As we ring in the new year, medical providers now can say goodbye to the CMS’s “Meaningful Use Incentive Program” (MU) and start preparing for the new Medicare incentive program.
Providers who used qualified systems in 2016 can still attest to Meaningful Use for the 2016 year (you must have been a “meaningful user” of certified electronic medical record system(s) for the minimum reporting period. Visit this website for more info on how the previous year’s MU programs worked and deadlines for attesting:
https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/2016ProgramRequirements.html
Now in 2017, Meaningful Use will become one of four components of the new “Merit-Based Incentive Payment System” or MIPS. MIPS is part of the bigger Medicare Access and CHIP Reauthorization or MACRA.2
The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) ended the Sustainable Growth Rate formula, which threatened clinicians participating in Medicare with potential payment penalties for 13 years.
The MACRA program introduces two paths that Medicare providers can choose from for participation:
- Advanced Alternative Payment Models (APMs) (providers apply for a special payment model program) or
- The Merit-based Incentive Payment System (MIPS) (a performance-based program)
Who Does this Affect?
Providers who are in an Advanced APM or who bill Medicare for more than $30,000 a year and care for more than 100 Medicare patients a year are affected. Providers with less than that are not affected and not part of the program.3 This includes:
- Physician
- Physician assistant
- Nurse practitioner
- Clinical nurse specialist
- Certified registered nurse anesthetist
Advanced Alternative Payment Models (APMs)
The Advanced APMs program allows certain providers to apply for the APM track. This gives added incentive payments to provide high-quality and cost-efficient care. See the APM website for more information on the special programs and how providers can apply:
https://qpp.cms.gov/learn/apms
The Merit-based Incentive Payment System (MIPS)
Most Medicare Providers will be part of MIPS. They will earn a payment adjustment based on evidence-based and practice-specific quality data submitted. According to CMS, the Quality Payment Program policy will reform Medicare payments for more than 600,000 clinicians across the U.S.
Providers participating in the program in 2017 will submit their data by March 2018 and based on submission, their 2019 Medicare payments will be adjusted up, down, or not at all.
MIPS is broken down into four categories and is setup so that the more Providers participate (and attest to), the higher score (and incentive) providers will get. A Medicare provider who does not participate at all (0%) may see up to 4% negative adjustment in 2019. A provider with a minimal amount of participation (e.g. submit one measure) may be able to avoid adjustment. For partial submission (submit the minimums for a partial year) they will see neutral or positive adjustments, and submit a full year and earn a positive payment adjustment.
|
CMS has setup a new website for the Quality Program4 which breaks down the four components of the MIPS:
- Quality (replaces PQRS) (60%)
- Improvement Activities (new) (25%)
- Advancing Care Information (replaces Meaningful Use) (15%)
- Cost (replaces Value Based Modifier) (0% in 2017)
|
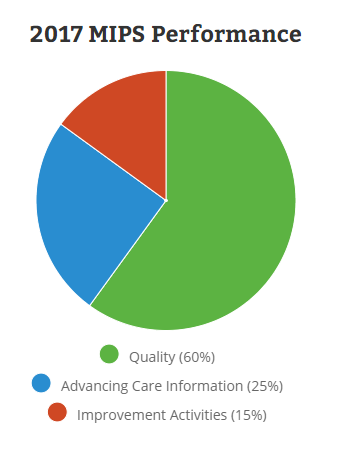
Image Credit: MIPS Quality Payment Program Website: https://qpp.cms.gov/measures/performance
|
If we take a quick look at how each category works:
- Quality
- Most Providers will report up to 6 quality measures (including an outcome type measure). Quality measures selected should be focused based on type of care and specialty as appropriate.
- Reporting period must be a minimum of 90 days.
- There are over 250 quality measures available, be sure to check your health record software system to see which ones they support (can help gather data for you) when planning.
- Measures go across many specialties and problem sets: For example, “Age Appropriate Screen Colonoscopy” – Report the percentage of patients greater than 85 years old seen by the Provider who received a colonoscopy screening Jan 1 to December 31.
- Improvement Activities
- Most Providers will attest to completing a minimum of 4 improvement activities for at least 90 days.
- As of the writing of this blog, the CMS tools shows 92 activities to choose from.
- Activities range from care coordination, patient safety changes and beneficiary engagements.
- Examples: Join and participate (for a minimum of 6 months) in your States Prescription Monitoring Program (PMP). Or another example: Engage patients, family and caregivers in developing a plan of care and prioritizing their goals for action, documented in the certified EHR technology.
- Advancing Care Information
- Use a qualified (certified) product (or products) for a minimum of 90 days.
- There are two different programs to pick from depending on your Electronic Health Record Software Certification. For 2017, you will be able to use either a 2014 Certified Product (previously called Stage 2 MU Certification) or a 2015 certified product (certified for the MU Final Ruling criteria). Attest to a minimum of:
- E-Prescribing
- Provide Patient Access
- Send Summary of Care Records
- Receive Summary of Care Records
- Report up to 9 additional measures for bonus credits
- Cost
- No actions required: Cost will be computing from your claims
- The cost category will be calculated in 2017, but will not be used to determine your payment adjustment. In 2018, CMS will start using the cost category to determine your payment adjustment.
Medicare Providers will want to research the programs and decide their level of participation early in 2017. Full year participation would require making sure their Electronic Health Record system is setup for minimal data tracking and other required features like E-Prescribing and Direct Messaging.
References
2017 Program requirements:
https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/2017ProgramRequirements.html
MIPS and MACRA:
http://www.impact-advisors.com/meaningful-use/mips-macra-mu-the-next-evolution-of-healthcare-payment-reform/#sthash.vMkVGSvN.dpuf
https://qpp.cms.gov/docs/Quality_Payment_Program_Overview_Fact_Sheet.pdf
MIPS Quality Payment Program Website:
https://qpp.cms.gov/measures/performance