
The world has seen remarkable changes this year, the methods we receive healthcare being a significant part of that change. COVID-19 has hurried the adoption of telemedicine into mainstream usage during the declared public health emergency (PHE). Legislation has now been presented to keep telemedicine as part of standard healthcare and make its usage more convenient for both providers and their patients.
President Trump’s Executive Order on Improving Rural Health and Telehealth Access
President Trump signed executive orders on August 3rd to promote the expansion of telehealth services. The Center for Medicare & Medicaid Services (CMS) outlined 135 services that are allowable via telehealth during the PHE, Trump’s executive order outlines that the services become permanently available via telehealth. The executive order also offers financial incentives for rural hospitals to continue seeing patients with a high-quality of care and directs the federal government to improve the healthcare communication infrastructure in rural areas.
“Telemedicine can never fully replace in-person care, but it can complement and enhance in-person care by furnishing one more powerful clinical tool to increase access and choices for America’s seniors,” said CMS Administrator Seema Verma. “The Trump Administration’s unprecedented expansion of telemedicine during the pandemic represents a revolution in healthcare delivery, one to which the healthcare system has adapted quickly and effectively. [1]
CMS Proposes Permanent Expansion of Telehealth
Consistent with Trump’s executive order, CMS proposed that many telehealth service payments should be expanded to be permanently covered past the PHE. A major hurdle hindering providers from adopting telemedicine as part of their practice is the disparity in the CMS reimbursement payment structure for in-person versus telehealth visits. During the PHE, CMS allowed parity in the payment structure for in-person and telehealth visits, making telehealth even more attractive for providers to participate. According to CMS, before the PHE, only 14,000 beneficiaries received a Medicare telehealth service in a week while in the last week of April, nearly 1.7 million beneficiaries received telehealth services.
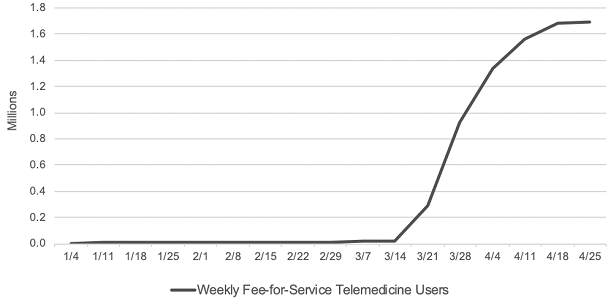
Source: CMS Health Affairs Blog. Internal CMS analysis of Medicare FFS claims data, March 17, 2020 through June 13, 2020(using data processed through June, 19, 2020) Notes: Telemedicine is defined to include services on the Medicare telehealth list including audio-only visits, as well as virtual check-ins and e-visits. https://www.healthaffairs.org/do/10.1377/hblog20200715.454789/full/
Many medical providers and associations have requested that the parity remain to allow telehealth to continue growing due to the better hold on financial security. CMS is asking for input from stakeholders regarding what services should bee added to the Medicare telehealth list and the public comment period for the proposed rule is open until October 5, 2020.
Temporary Reciprocity to Ensure Access to Treatment (TREAT) Act
Another barrier that providers are facing is the lack of inter-state licensing ability to be able to practice telemedicine for patients residing in other states. Senators Chris Murphy and Roy Blunt have presented the Temporary Reciprocity to Ensure Access to Treatment (TREAT) Act which would grant providers the ability to treat patients in any state during and immediately following the PHE. The Act also establishes that the reciprocity can be reactivated should another PHE happen in the future, again reducing inter-state complications. Some states have reduced the requirements for providers to get a license, and some states have granted temporary licenses. Mandating nation-wide reciprocity could be invaluable to patient health during a worsening or future PHE.
DEA Telehealth Policies
EPCS
The Ryan Haight Act of 2008 established regulations and prohibited healthcare providers from prescribing controlled substances to patients that they haven’t first examined in-person. Section 802(54)(D) of the Controlled Substances Act allows for the Ryan Haight Act to be circumvented during a public health emergency which the DEA invoked on March 16, 2020. This currently allows MDToolbox users to electronically prescribe controlled substances (EPCS) for patients via telemedicine. Patients must be evaluated using a real-time, two-way, audio-visual communications device.
The DEA has missed several deadlines to establish rules and a waiver system to allow electronic prescribing of controlled substances via telemedicine when there is not a PHE. Reducing these road-blocks, as we are seeing with the emergency measures in place due to COVID-19 can help bring healthcare into the 21st century and help reduce stress on our medical system and patients.
DEA State Registration
The DEA has also waived the requirement for state-specific registrations during the PHE. The exception to separate registration requirements across state lines was issued March 25, 2020 and allows prescribers who are registered in at least one state to prescribe controlled substances to patients in other states via telemedicine.
Opioid Use Disorders
The DEA, in partnership with the Substance Abuse and Mental Health Services Administration (SAMHSA), also stated that it is allowing authorized providers to prescribe buprenorphine to new and existing patients with Opioid Use Disorder (OUD) via only telephone voice calls without first requiring an examination of the patient in person or via telemedicine. This exception is only during the PHE and prescribing practitioners must be DATA-waived.
Telehealth Response for E-prescribing Addiction Therapy Services (TREATS) Act
Not to be confused with the earlier mentioned TREAT act, the Telehealth Response for E-prescribing Addiction Therapy Services (TREATS) Act was introduced by Senators Rob Portman (R-OH) and Sheldon Whitehouse (D-RI) and looks to make some of the telehealth substance use disorder (SUD) treatment changes permanent.
The bill adds to and replaces language in the current Telehealth for Substance Use Disorder Treatment codes. The changes would allow a Schedule III or IV medication to be prescribed for the purpose of treatment for an Opioid Use Disorder via “1 in-person medical evaluation or 1 telehealth evaluation”. The bill then clarifies that the “1 telehealth evaluation” shall not be construed to imply that a single telehealth evaluation demonstrates the usual course of professional practice. The medical provider will need to continue follow-up and management of the patient and medication after the initial in-person or telehealth visit per current guidelines.
Continue to follow our blog and social media for information related to telehealth and electronic prescribing. MDToolbox looks forward to providing tools and resources to assist telemedicine providers throughout the United States to ease the transition, helping our customers increase the efficiency of their office and combat the opioid epidemic. With MDToolbox, prescribers have access to tools such as Electronic Prescribing of Controlled Substances (EPCS), the ability to check most State’s PMPs without having to separately login to their State portal, and convenient on the go e-prescribing with our mobile app! We offer a free 30 day free trial, so Contact us for more information!
[1]https://www.cms.gov/newsroom/press-releases/trump-administration-proposes-expand-telehealth-benefits-permanently-medicare-beneficiaries-beyond