
----- Blog Index / The Evolution of EPCS [MDToolbox Staff] [2014-01-27]
Electronic prescribing of controlled substances (EPCS) is just starting to gain ground. The Drug Enforcement Agency (DEA) rule allowing prescribers to electronically write prescriptions for controlled substances actually went into effect over three years ago. However, its adoption has been slow.
The need to be able to send controlled substances electronically is definitely there. Approximately 11% of all prescriptions written are for controlled substances and 90% of prescribers write prescriptions for such drugs1. If these prescriptions can’t be sent electronically, prescribers must handwrite or print them. This can be a big interruption and slowdown in a prescriber’s workflow. In addition, EPCS increases safety and decreases fraud. So why is it taking so long for EPCS to become a norm?
The DEA’s Interim Final Rule (IFR) first approved electronic sending and receiving of controlled substances in March 2010, and it went into effect on June 1, 2010. Though in order to actually use EPCS, prescribers, e-Prescribing software, and pharmacy software must meet strict regulations. Prescribers are required to go through stringent identity proofing and receive two-factor authentication credentials. Each time they send a controlled substance, prescribers must use their two-factor authentication. The two-factor has to be two of the following three items:
1) Something only the prescriber KNOWS, like a password or an answer to a challenge question
2) Something the prescriber IS, biometric data such as a fingerprint or
3) Something the prescriber HAS like a device or token separate from the computer he is prescribing on.
E-Prescribing software and pharmacy software must go through a DEA Certification or third-party audit to verify they comply with the regulations. The IFR requires e-Prescribing software to have a two-factor authentication protocol, have access controls so only prescribers with the proper permissions can send controlled substance prescriptions, and put extra security and auditing measures in place. Pharmacy software is also required to have access controls and stricter security measures, as well as additional features to be able to receive electronic controlled substance prescription orders.
In addition to the Federal DEA requirements, each state has their own laws and regulations concerning EPCS. While 47 states have approved EPCS, there is still a small percentage of pharmacies in each of these states that are able to receive electronic prescriptions for controlled substances.
It is clear that meeting all of these requirements can be challenging and takes ample time and money for all involved. This has caused the progression to be quite slow. Here’s how it looks on a year-by-year basis:
2010: DEA IFR goes into effect and states begin aligning their rules with those of the DEA.
2011: Software vendors working to meet requirements.
2012: First e-Prescribing and pharmacy software vendors certified. Surescripts® reports a “modest number of EPCSs” transmitted in eight states as of May 2012.
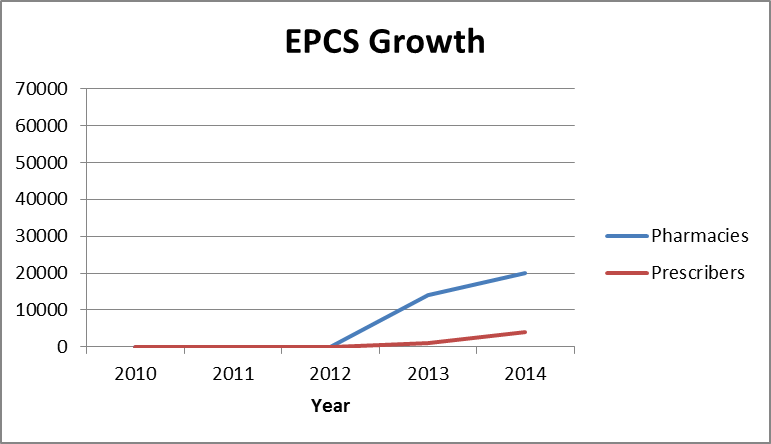
2013: About 14,000 pharmacy stores signed up for EPCS in 44 states, but only about 1,000 prescribers nationwide using EPCS as of Mid-2013.
2014: About 20,000 pharmacy stores signed up for EPCS in 47 states. However, only 14 e-Prescribing systems certified for EPCS out of over 600 prescribing applications on the Surescripts® network. 2

Out of almost 70,000 total pharmacies and more than half a million e-Prescribing prescribers, the numbers of those using EPCS are still small. However, that’s changing as EPCS growth takes off.
As one of the 14 certified Prescriber EPCS systems, MDToolbox is at the forefront of the EPCS movement. We see now as the time that EPCS is going to take off. MDToolbox is offering both standalone EPCS for prescribers and EPCS plug-in modules for EHRs looking for a quick and inexpensive way to get on-board with the movement. With laws like New York’s I-STOP mandating ALL prescriptions be sent by March 27, 2015, we believe EPCS will see huge growth in 2014.