The Centers for Medicare & Medicaid Services (CMS) issued their final ruling for improving and modernizing the Medicare Part C (Medicare Advantage) and Part D programs on May 16th, 2019. This 201-page document primarily focuses on implementing changes that will save money for patients as healthcare and prescription costs continue to rise.
The costs of oral brand-name drugs increased annually by 9.2% from 2008 to 2016, while oral specialty drugs increased 20.6%[1]. These numbers are only expected to continue rising, putting additional strain on patient budgets. The national average inflation rate is roughly 2%, therefor prescription drugs are increasing 5-10 times as rapidly as the cost of living.
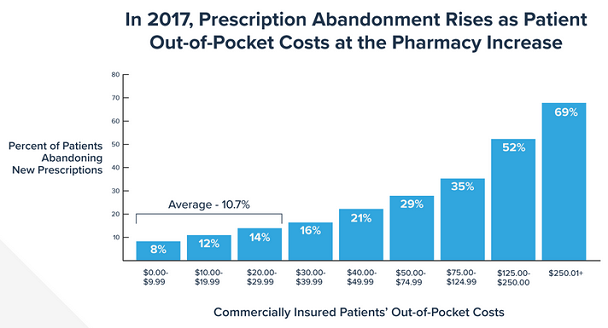
The price-tiered chart above shows how often prescriptions are being abandoned at the pharmacy due to drug prices. An average of 10.7% of prescriptions under $30 are abandoned, while 69% of prescriptions over $250 are abandoned.[2] A large percentage of those patients who abandoned their prescriptions do not follow-up with their medical provider for another potentially cheaper alternative. Some patients may also take their medications off-label by skipping days, potentially resulting in even higher future healthcare costs. Prescription non-adherence has been estimated to produce up to $300 billion in avoidable healthcare costs per year in the US.[3]

https://www.healthcarefinancenews.com
The CMS ruling contains many updates to Medicare policy including:
- Forcing sponsors to include formulary for all 6 categories/classes of drugs. There was a proposed exception to exclude a class of drug from their formulary if the price rose too high, this exception was not included in the ruling.
- Part D EOBs must contain information on drug price increases and include alternative therapy options and their pricing to better inform patients of possible ways to have a lower out of pocket cost
- The ruling prohibits sponsors from issuing “gag clauses” to pharmacies, which previously prohibited the pharmacy from advertising a cheaper cash price for a prescription.
- There are additional regulation changes for Step Therapy requirements with Medicare Advantage on Part B drugs.
- The final part of this ruling focuses on giving patients and prescribers better access to prescription drug pricing via their EHR or electronic prescribing system.
The CMS ruling will require that Medicare Part D sponsors adopt a tool that displays real-time price benefit information that is capable of integrating into e-prescribing software by January 1st, 2021. This means the tool must be functional by the deadline but not necessarily available in EHRs or e-prescribing software yet. As of the date this blog was published, many of the EHRs and e-prescribing software on the market do not have the ability to show real-time pricing/benefit information at the point of prescribing. Some vendors do have the ability so long as the feature has been added and the patient’s particular insurance plan has made that information available to EHRs. For example, MDToolbox is proud to have the ability to display patient formulary information, drug prices, and preferred drug levels for patients on plans that support it. The number of patients with accessible Medicare Part D pricing will continue to grow as the plan sponsors are required to make their pricing available.
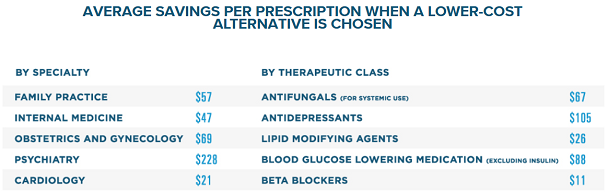
Surescripts, a health information network hub that digitally connects healthcare providers to pharmacies and PBMs/insurance companies recently released their 2018 research showing that being able to view the real-time benefits caused prescribers to change their medication selections 28% of the time, actively saving the patient money. The average cost saving per prescription written by a family practice in 2018 was $57, the average saving for a psychiatry office was $228 per prescription![4]
The increase in usage of Electronic Prescribing throughout the US means better protections and convenience for both prescribers and patients. Real-Time Benefit Pricing will save patients money and help keep their satisfaction in their medical providers. We look forward to working with providers throughout the US to better empower themselves, better inform their patients, and help provide tools and resources for making their practice more efficient. With MDToolbox providers have access to tools such as Electronic Prescribing of Controlled Substances (EPCS) and convenient on the go mobile e-prescribing. Contact us for more information or to start a free 30 day free trial.
[1]https://www.healthaffairs.org/doi/abs/10.1377/hlthaff.2018.05147
[2]https://catalyst.phrma.org/69-percent-of-patients-abandon-medicines-when-cost-sharing-is-more-than-250
[3]https://www.pharmacytimes.com/contributor/timothy-aungst-pharmd/2018/06/does-nonadherence-really-cost-the-health-care-system-300-billion-annually
[4]https://surescripts.com/news-center/national-progress-report-2018/